For the treatment of COVID-19 positive cases, on 21st June 2020, Hyderabad based pharmaceutical company -Hetero Drugs has received approval from the Drug Controller General of India (a department under- CDSCO: Central Drugs Standard Control Organization) for manufacturing and marketing of antiviral drug Remdesivir.
Brandname for Marketing
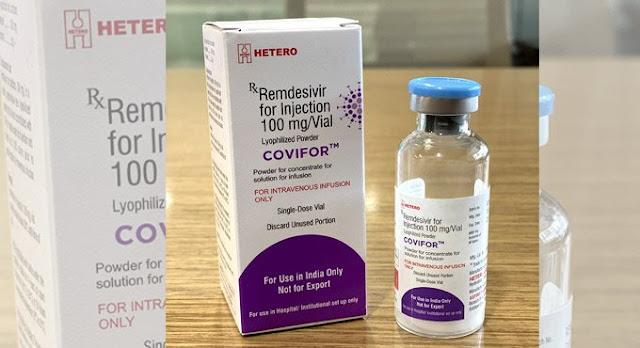
- Under the brand name ‘Covifor’, Hetero Pharmaceuticals’ generic version of Remdesivir will be marketed in India.
- The drug is manufactured at the Hetero company’s Hyderabad facility, while its Active Pharmaceutical Ingredients (API) is being developed at Andhra Pradesh’s Visakhapatnam.
Remdesivir
- The antiviral drug ‘Remdesivir’ is administered via injection into a vein.
- This antiviral medication was developed by the United States biopharmaceutical company Gilead Sciences for treatment of Hepatitis C.
- United Kingdom, Singapore, and Japan are the countries where the drug has been approved to date for the treatment of COVID-19 cases.
Price and Availability
- As it is an injectable drug, it has to be administered under the supervision of a healthcare practitioner and will not be available through any retail channel.
- The drug will be made available for hospitals and government healthcare agencies.
- It will be available in a 100mg injectable dose.
- The price will be in the range of Rs 5,000 to 6,000 per dose.
- Hetero has targeted to manufacture one lakh doses of it in the upcoming weeks, the company has stated that production can be increased based on the demand.
- Covifor will be used for the treatment of confirmed COVID-19 cases for those who have severe symptoms of the disease.
No comments:
Post a Comment